Real world study: Safety & effectiveness of IN esketamine for treatment-resistant depression
Journal of Comparative Effectiveness Research | 04 November 2022
Thanks to all who contributed to our latest paper—especially Madeline Brendle, our incredible pharmacotherapy outcomes grad student and data scientist extraordinaire. Seeing this project complete to publication was quite the process but well worth it, and we’re very excited to be sharing the results. Check out the details below.
Background & Aims: There is limited real-world evidence for patients with treatment-resistant depression (TRD) receiving esketamine nasal spray.
Methods: This retrospective cohort study used data collected from a psychiatric clinic’s EHR system.
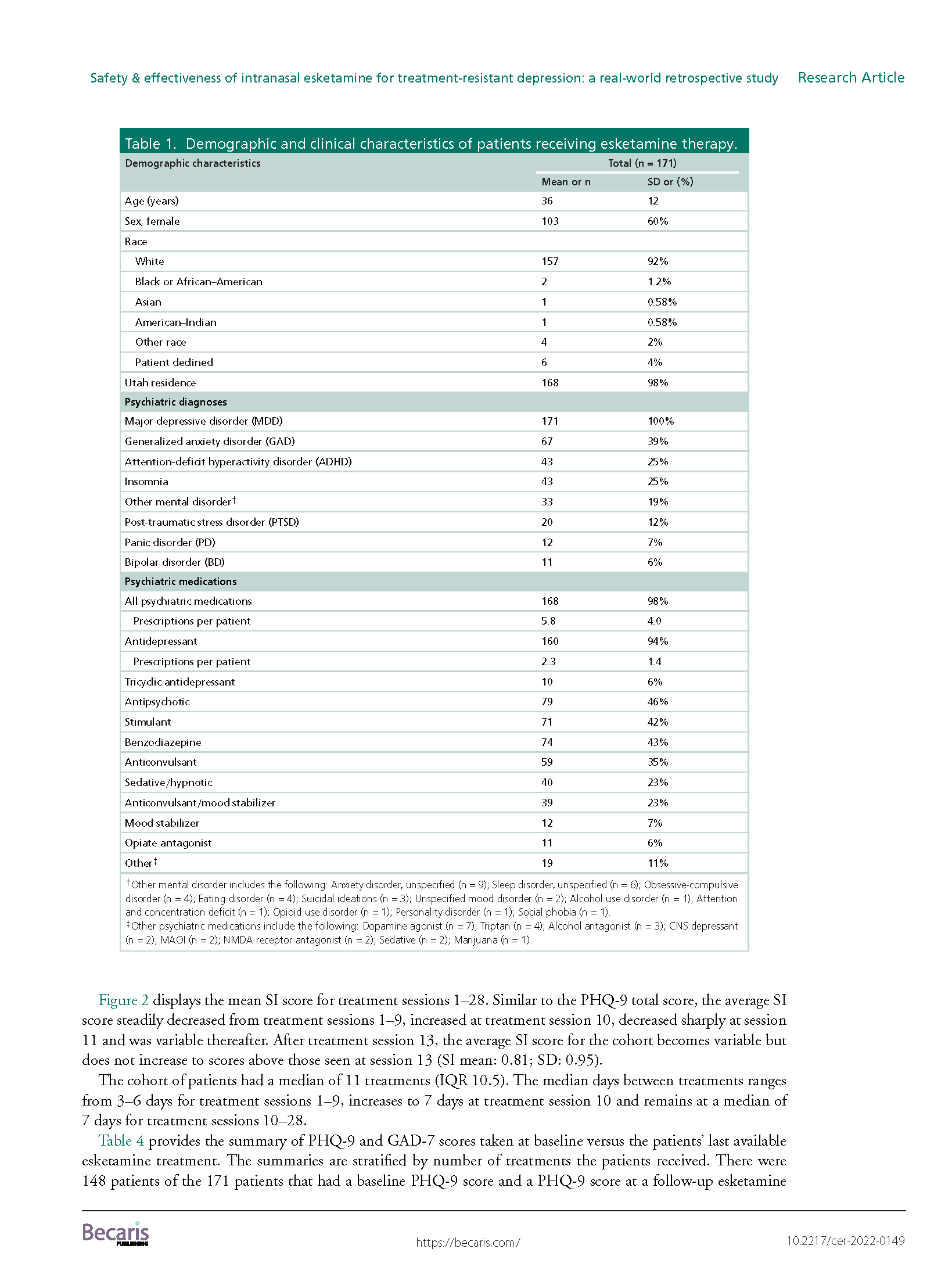
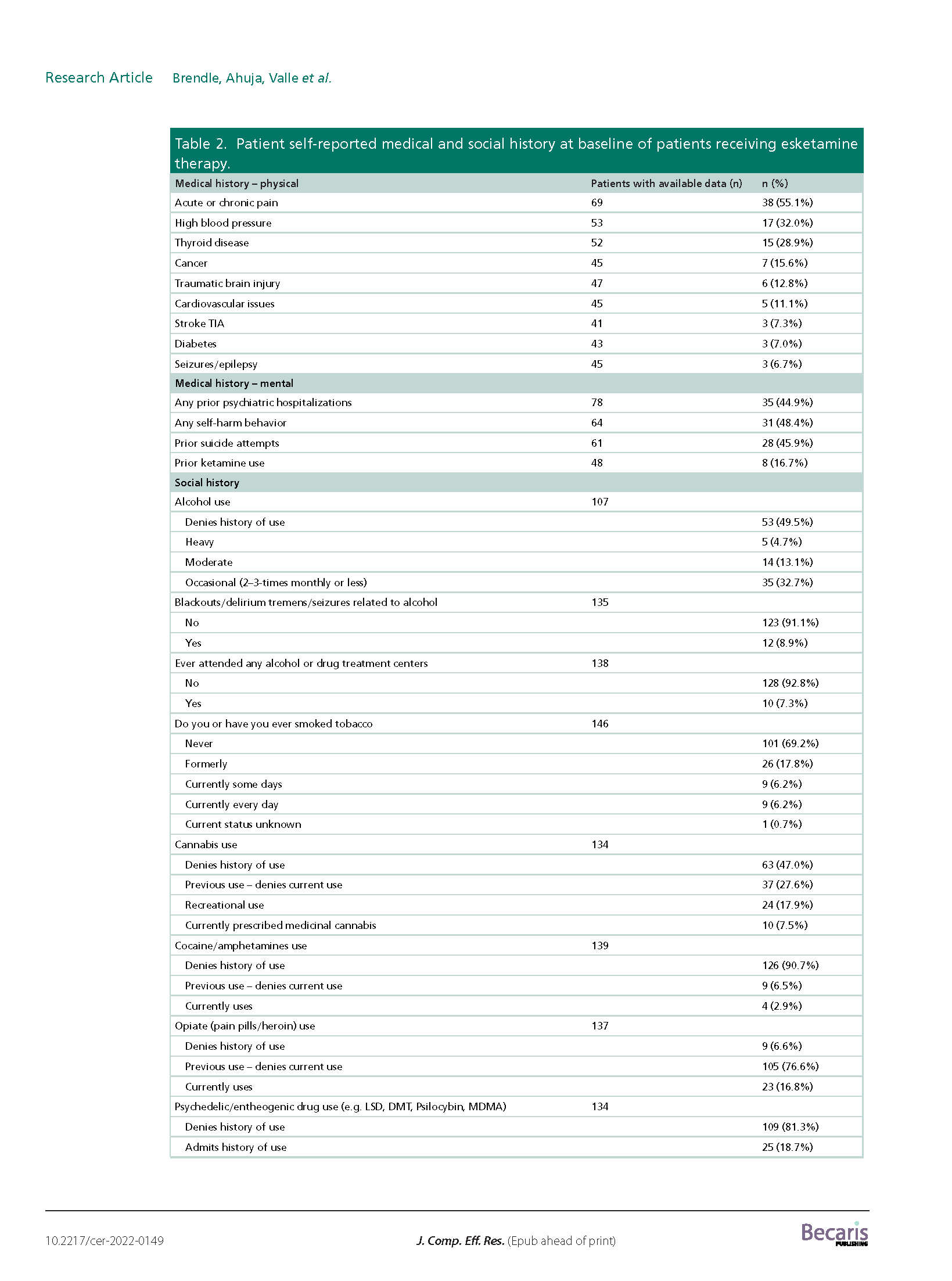
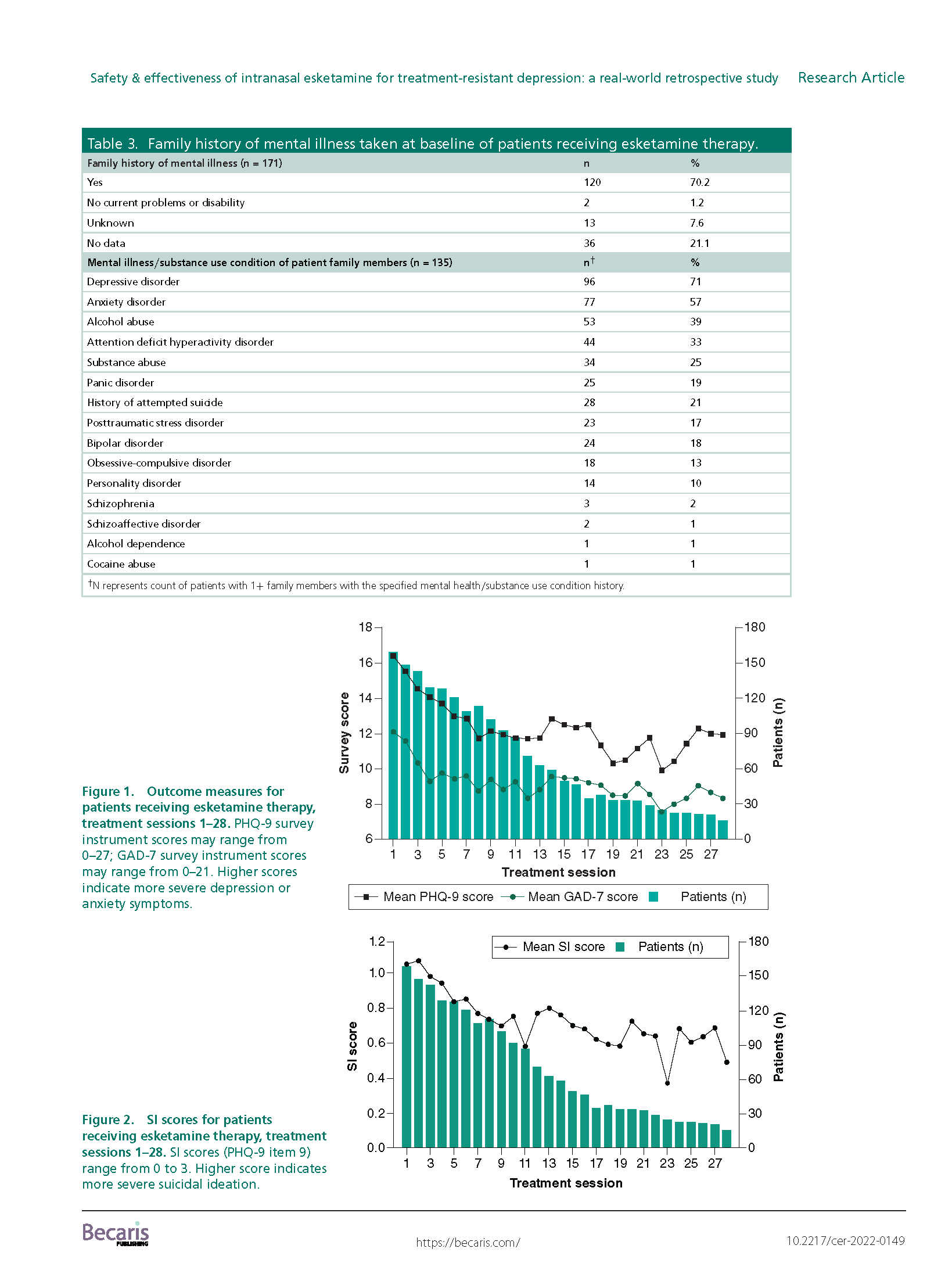
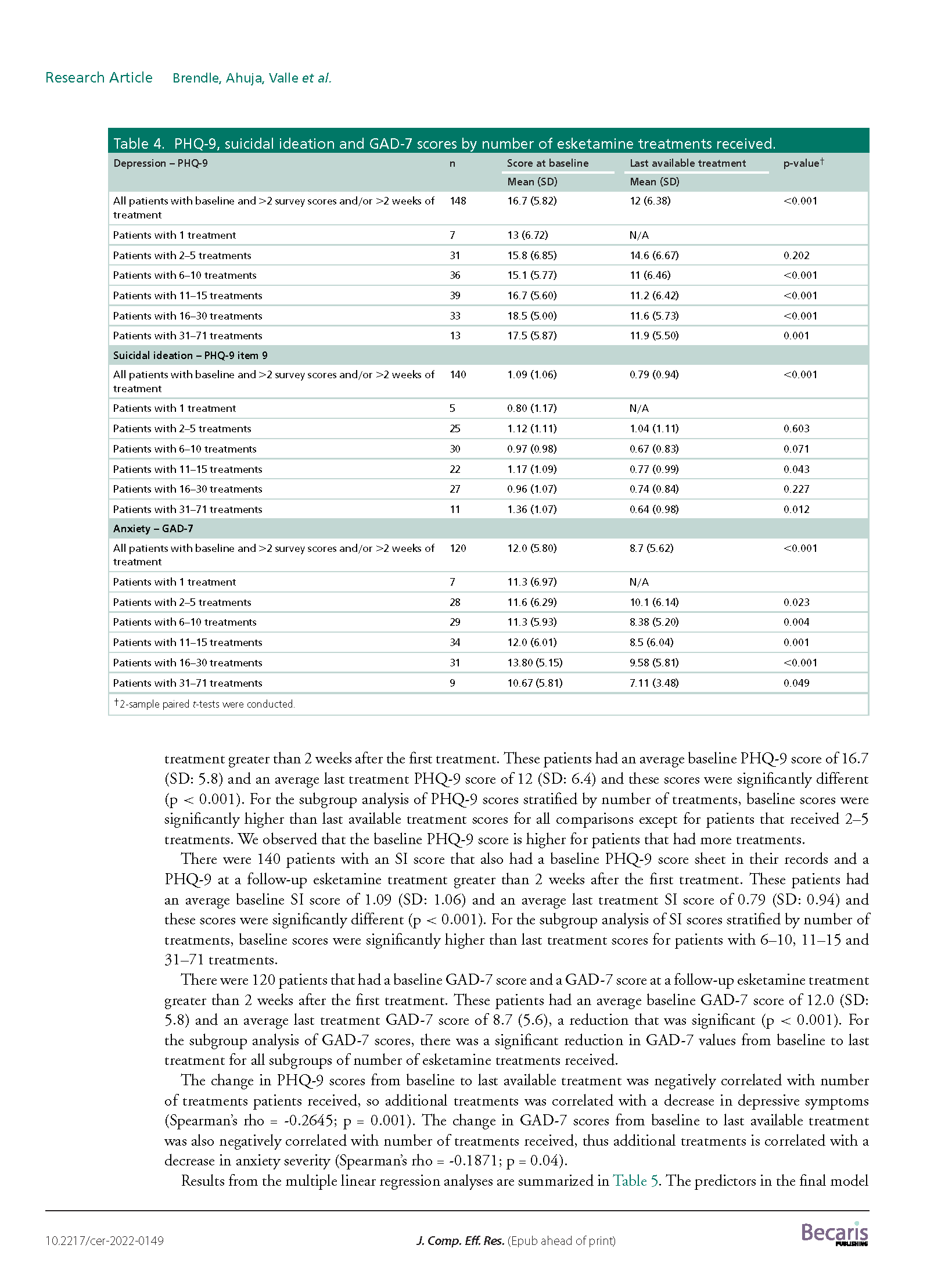
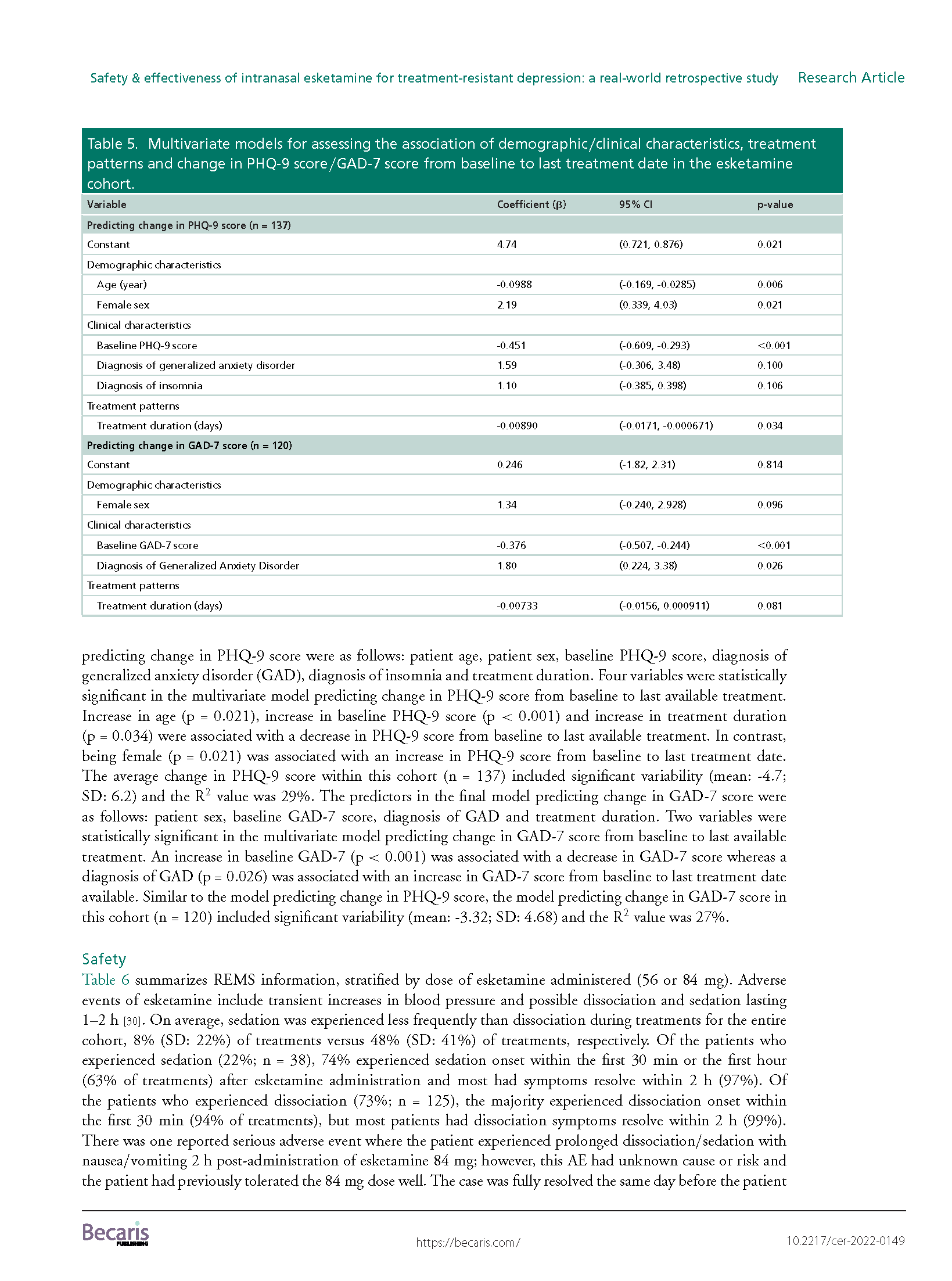
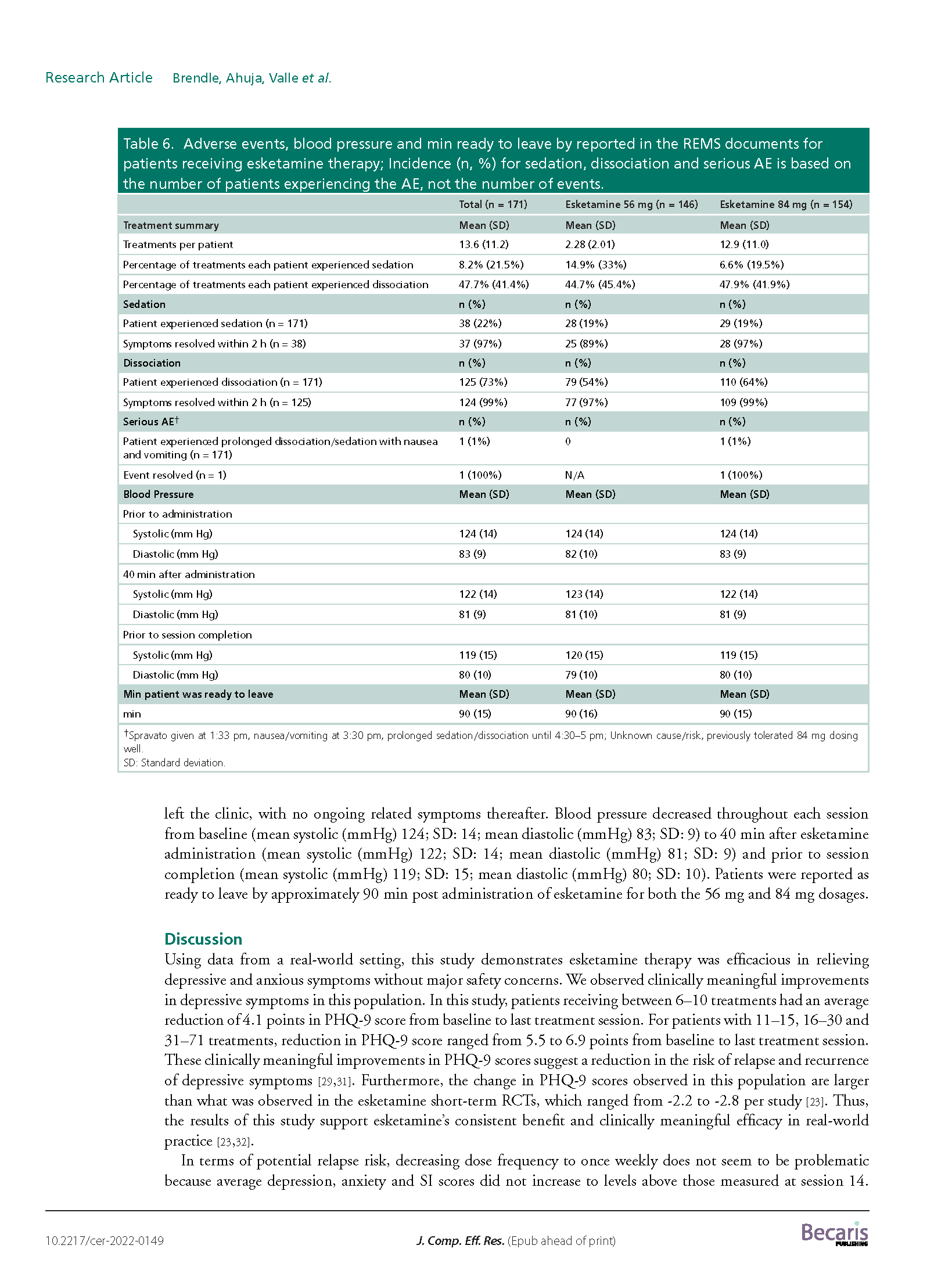
Results: A total of 171 TRD patients received esketamine July 2019–June 2021. This predominantly female, white population had several mental health comorbidities and high exposure to psychiatric medications. We observed significant reductions (p < 0.001) in average PHQ-9 and GAD-7 scores from baseline (PHQ-9: mean: 16.7; SD: 5.8; GAD-7: mean: 12.0; SD: 5.8) to last available treatment (PHQ-9: mean: 12.0; SD: 6.4; GAD-7: mean: 8.7; SD: 5.6). There were no reports of serious adverse events.
Conclusions: This study found a significant disease burden for patients with TRD. Esketamine appears to be well tolerated and effective in improving depression and anxiety.








Citation
Brendle M, Ahuja S, Valle MD, et al. Safety and effectiveness of intranasal esketamine for treatment-resistant depression: a real-world retrospective study. J Comp Eff Res. 2022;11(18):1323-1336. doi:10.2217/cer-2022-0149